Professor of Plant Biology and Executive Director, UC Botanical Garden
Ph.D. Biology Harvard University, 1975
B.S. Botany University of California, Davis, 1967
The Feldman Lab broadly researches plant development, with an emphasis on roots and the root meristem, including the root cap. We have focused much of our work on a population of cells known as the quiescent center and have shown that many of its activities and characteristics are related to redox status. For this effort we have developed a redox-sensing GFP which allows us to measure, in real time, redox status. We hypothesize that many of the profound effects and controls of auxin on root development are associated with changes in redox status. We also use microarrays to characterize distinct root meristem populations, including the root cap, in which we study gravity signal transduction. A major goal of all of our work is to link molecular changes with biochemistry.
A second major emphasis involves developing biosensors to allow plants to act as reporters of their own physiological status. We have focused on the development of biosensors for heat stress. Our long term goal is to improve crop yield by having the plant "tell" us when it needs some alteration of its growing conditions (for example, cooler temperatures, or water), before it experiences stress, and thus reduction in yield.

Our lab's interests are in the area of plant development, with an emphasis on meristems, particularly those of roots. We are investigating how the various populations of cells which comprise or surround the meristem interact to control root development, especially patterning. Much of our recent effort has focused on two populations of cells: the root cap, and a region of mitotically inactive cells known as the quiescent center. We have shown that quiescent center formation precedes the organization of a root meristem and have hypothesized that a quiescent center is necessary for meristem organization. Based on characteristics of cells comprising the quiescent center we believe that these cells are true “stem cells” in the animal sense. Much of our current work is directed at understanding the underlying molecular and physiological controls of this “stemness”.
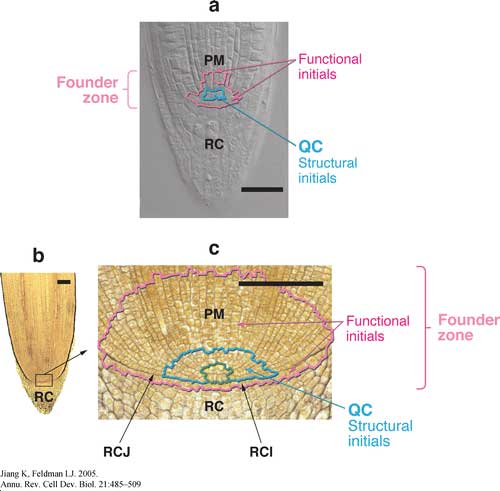
Pictured below are root apical meristems of (a) Arabidopsis thaliana and (b,c) Zea mays showing the location of various cell populations. For b and c, note the convergence of cell files to a small number of cells, circumscribed in blue-green (the originally designated promeristem). QC, quiescent center; PM, proximal meristem; RC, root cap; RCI, root cap initials; RCJ, root cap junction. Scale bar = 100 m.
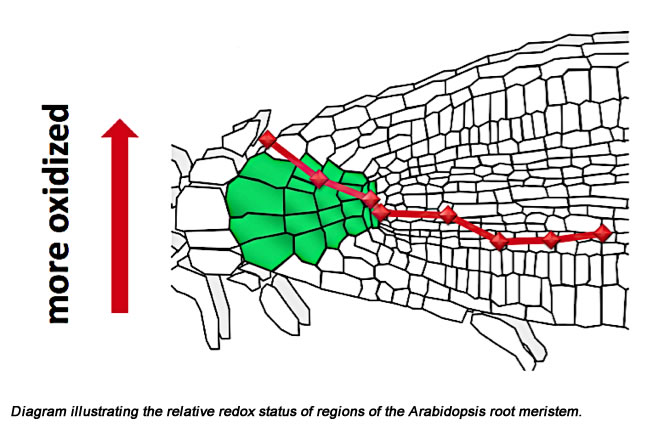
It is now widely agreed that the local accumulation of auxin at the embryo pole is THE formative event in the establishment of a quiescent center (a stem cell niche), around which the root meristem organizes. Linking auxin accumulation and meristem establishment is currently a major focus in the lab. We believe that an important intermediate step in meristem establishment is an auxin-induced change in redox status in the presumptive quiescent center. In order to monitor hypothesized auxin-induced changes in redox status we have developed a GFP which when irradiated with two different wavelengths of light provides a ratiometric measurement of redox status in specific regions/cells in the root (Plant Physiology 141: 397-403). Using this new tool we have shown that the quiescent center maintains a relatively oxidizing (redox) microenvironment and that this is accompanied by low levels in the quiescent center of the two major redox regulators in organisms, gluthathione and ascorbic acid. By altering the redox status, making it relatively more reducing, we are able to stimulate cells in the quiescent center to divide and to lose their stem cell characteristics.
In addition to exploring the role of redox status in root meristem development and physiology we are using various GFP marker lines specific to the quiescent center, as well as microarrays, to examine molecular events underlying quiescent center (and root meristem) formation. Current efforts are focusing not only on the quiescent center but also on a zone of meristem cells, the "transition zone", in which cells lose their meristematic properties, stop dividing, and begin to elongate and differentiate. Towards an understanding of the dynamics of the transition zone, we are currently focusing on a family of transcription factors, the GATA family, members of which have specific expression patterns in the transition zone.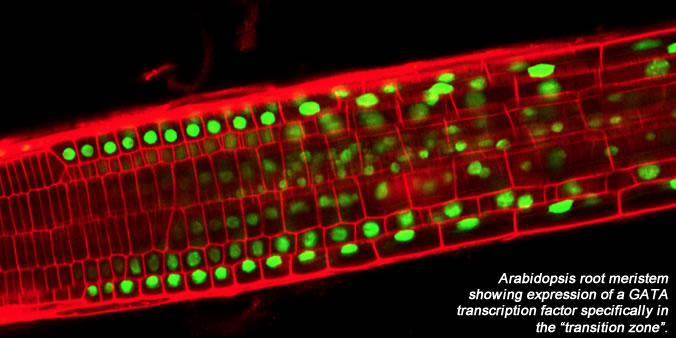
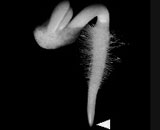
We also are investigating the root cap, which is the terminal-most population of cells in a root (Arrow points to the root cap of a radish seedling). The root cap is the site of perception for many environmental stimuli, including gravity and light. Hence, it has much of its physiology and biochemistry devoted to the perception and transduction of environmental stimuli. We investigate how the cap communicates with the rest of the root. The cap also represents a unique population of cells which, in a space of 0.5 mm, initiates, differentiates, and then releases cells to the external environment where these detached cells may continue to live for up to 7 days. Using microarrays, we are studying the many original and unique differentiation events occurring in the cap and have examined many unique activities in the cap including it ability to sense many environmental stresses and stimuli, including gravity. This work has been recently described in, Transcription Profile Analyses Identify Genes and Pathways Central to Root Cap Functions in Maize (2005) Keni Jiang, Shibo Zhang, Stanley Lee George Tsai, Kyungpil Kim, Haiyan Huang Tong Zhu and Lewis J. Feldman. Plant Molecular Biology 60: 343-360.
Our most recent project aims to engineer plants which can act as their own reporters (bioreporters) for various environmental stresses. Initially we are focusing on heat and salt stress. Using GFP as our reporter we have engineered plants to fluoresce when exposed to various environmental stresses. This "output" from the plant can be measured with a portable meter (pictured below) and can then be linked directly to greenhouse environmental control systems, in order to moderate the stressful environment.

With regard to graduate student research, I encourage graduate students to select and develop their own research projects. This increases the diversity of projects being done in the lab and makes for a broader graduate research experience. Graduate student projects have included: studies of the transition to flowering in Arabidopsis; physiology and development of the peanut gynophore; the genetic and molecular basis for leaf shape in Arabidopsis. Current graduate student research involves characterizing the role of the cle family of genes in Arabidopsis root development.
Jiang, K. and Feldman, L.J. (2005) Regulation of Root Apical Meristem Development. Annu Rev Cell Dev Biol. 2005 Vol. 21: 485-509 pdf 540 kb
Jiang, K, Zhang, S, Lee, S, Tsai, G, Kim, K, Huang, H, Zhu, T, and Lewis J. Feldman, L.J. (2005) Transcription Profile Analyses Identify Genes and Pathways Central to Root Cap Functions in Maize. Plant Molecular Biology 60: 343-363.
Jiang K. Ballinger T, Li, D, Zhang, S, Feldman LJ (2006) A Role for Mitochondria in the Establishment and Maintenance of the Maize Root Quiescent Center. Plant Physiol. 140: 1118-1125.
Jiang, K, Schwarzer, C, Lally, E, Zhang, S, Ruzin, S, Machen T, Remington, SJ and Feldman LJ . (2006). Expression and characterization of a redox-sensing Green Fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol. 141: 397–403.
Kim K, Zhang S, Jiang K, Cai L, Lee I-B, Feldman L.J, Huang H 2007. Measuring similarities between gene expression profiles through new data transformations. BMC Bioinformatics, 8:29-43.
Jiang K, Zhu T, Diao Z, Huang H, Feldman LJ 2010. The maize root stem cell niche: a partnership between two sister cell populations. Planta 231, 411-424
Rosenwasswer S, Rot I, Meyer AJ, Feldman L, Jiang K, Friedman H. 2010 A fluorometer-based method for monitoring oxidation of redox-sensitive GFP (roGFP) during development and extended dark stress. Physiologia Plantrum 138, 493-502.
De Tullio MC, Jiang K, Feldman L 2010. Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiology and Biochemistry 48, 328-336.
Meng L, Wong JH, Feldman LJ, Lemaux PG, Buchanan BB 2010. A membrane-associated thioredoxin required for plant growth moves from cell-to-cell suggestive of a role in intercellular communication. PNAS 107, 3900-3905.
Jubany-Mari T, Alegre-Battle L, Jiang K and Feldman LJ 2010. Use of a redox-sensing GFP(c-roGFP1) for real-time monitoring of cytosol redox status in Arabidopsis thaliana water-stressed plants. FEBS Letters 584, 889-897.
Jiang K, Feldman LJ. 2010. Positioning of the auxin maximum affects the character of cells occupying the root stem cell niche. Plant Signaling and Behavior 5, 1-3.
Meng L, Feldman LJ. 2010. The roles of different CLE domains in Arabidopsis CLE polypeptide activity and functional specificity. Molecular Plant 3, 760-772.
Meng L, Feldman, LJ. 2010. CLE14/CLE20 peptides may interact with CLAVATA2/CORYNE receptor-like kinases to irreversibly inhibit cell division in the root meristem of Arabidopsis. Planta (2010) 232:1061–1074.
Jun, J.H., Fiume, E., Roeder, A.H.K., Meng, L., Sharma, V.K., Osmont, K.S., Baker, C., Ha, C.M., Meyerowitz, E.M., Feldman, L.J. and Fletcher, J.C. 2010. Comprehensive analysis of CLE polypeptide signaling gene expression and over- expression activity in Arabidopsis. Plant Physiol. 154: 1721-1736.
Meng L, Buchanan BB, Feldman LJ, and Luan S. 2012. CLE-like (CLEL) peptides control the pattern of root growth and lateral root development in Arabidopsis. Proceeding of the National Academy of Sciences, in press. www.pnas.org/cgi/doi/10.1073/pnas.1119864109
Stonebloom S, Brunkard, JO, Cheung AC, Jiang K, Feldman LJ and Zambryski PC. 2012 Redox states of plastids and mitochondria differentially regulate intercellular transport via plasmodesmata. Plant Physiology, in press
Kim K, Jiang K, Teng S-L M; Lewis J. Feldman LJ, Huang, H. 2012 Using biologically interrelated experiments to identify pathway genes in Arabidopsis. Bioinformatics 2012; doi: 10.1093/bioinformatics/bts038
Meng L., Buchanan B.B., Feldman L.J., Luan S. 2012 A putative nuclear CLE-like (CLEL) peptide precursor regulates root growth in Arabidopsis Molecular Plant, in press.
Szymanowska-Pulka j, Potocka I, Karczewski J, Jiang K, Nakielski J, Feldman LJ. 2012 Principal growth directions in development of the lateral root in Arabidopsis thaliana. Annals of Botany 110, 491-501.
Brossa R, Pinto-Marijuan M, Jiang K, Alegre L, Feldman LJ 2013. Assessing the regulation of leaf redox status under water stress conditions in Arabidopsis thaliana. Plant Signaling and Behavior 8, 1-11.
Hines G, Modavi C, Jiang K, Packard A, Poolla K, Feldman LJ 2015 Tracking transience: a method for dynamic monitoring of biological events in Arabidopsis thaliana biosensors. Planta 242, 1251-1261.
Wang R Y X, Jiang K, Feldman LJ, Bickel P, Huang H. 2015 Inferring gene-gene interactions and functional modules using sparse canonical correlation analysis. The Annals of Applied Statistics 9, 300-323.
Jiang K, Moe-Lange J, Hennet L, Feldman LJ 2016 Salt Stress Affects the redox status of Arabidopsis root meristems. Frontiers in Plant Science 7, 1-10
Jiang, K, Yung V, Chiba T, Feldman LJ, 2017. Longitudinal patterning in roots: a GATA2-auxin interaction is associated with the formation and maintenance of the transition domain. Planta, in press.
Distinguished Teaching Award - University of California, Berkeley - 1996
CNR Teaching Award - College of Natural Resources - 1992
Courses
Webcast to Bio 1b, SPRING 2012
Lewis J. Feldman
Berkeley, California 94720